Abstract: Cervical cancer remains a major cause of morbidity and mortality among women, especially in the developing world. Increased synthesis of proteins, lipids and nucleic acids is a pre-condition for the rapid proliferation of cancer cells. We show that scanning near-field optical microscopy, in combination with an infrared free electron laser (SNOM-IR-FEL), is able to distinguish between normal and squamous low-grade and high-grade dyskaryosis, and between normal and mixed squamous/glandular pre-invasive and adenocarcinoma cervical lesions, at designated wavelengths associated with DNA, Amide I/II and lipids. These findings evidence the promise of the SNOM-IR-FEL technique in obtaining chemical information relevant to the detection of cervical cell abnormalities and cancer diagnosis at spatial resolutions below the diffraction limit (≥0.2 μm). We compare these results with analyses following attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy; although this latter approach has been demonstrated to detect underlying cervical atypia missed by conventional cytology, it is limited by a spatial resolution of ~3 μm to 30 μm due to the optical diffraction limit.
Introduction
Cervical cancer is associated with the persistent infection of high-risk types of human papillomavirus (HPV), together with other socioeconomic co-factors1. Screening involves cytological and histological classification of cervical cells. In the UK, cytological examination of cervical squamous cells is classified as normal, borderline or mild dyskaryosis, moderate or severe dyskaryosis and invasive cervical cancer (ICC). Histology is defined as normal, cervical intra-epithelial neoplasia (CIN): CIN1, CIN2, CIN3, or invasive cervical cancer. For atypical cells found in the glandular cells of the cervix, the pre-invasive lesion of adenocarcinoma is defined by changes termed cervical glandular intraepithelial neoplasia (CGIN), and sub-classified as low-grade cervical glandular intra-epithelial neoplasia (LGCGIN) and high-grade cervical glandular intra-epithelial neoplasia (HGCGIN). Squamous and glandular lesions may co-exist together and are defined by the level of CIN together with either LGCGIN or HGCGIN. Conventional screening is flawed as it is dependent on the subjective visual inspection of cytology; this often results in mis-diagnoses when grading samples2.
Attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy has shown potential over conventional screening methods, demonstrating it can segregate grades of cervical cytology more accurately than conventional cytological screening3,4, classify cervical cytology based on HPV infection with low- or high-risk types5, and can diagnose underlying disease more accurately that conventional cytology screening2. However, FTIR spectroscopy is limited in spatial resolution by the effect of diffraction, defined as the interference of waves when they hit an obstacle or slit. This effect restricts the spatial resolution of FTIR to about half the wavelength of light or ~3 μm to 30 μm6, with the resolution being a measure of how closely the lines of an image can be resolved (i.e., the number of independent pixels per value per unit length).
Scanning near-field optical microscopy (SNOM) belongs to a family of nanoscopic techniques that have shown potential in providing detailed information on cell topography and cytoplasmic structures. SNOM has a clear advantage over conventional infrared (IR) microscopy in terms of spatial resolution because it is able to overcome the diffraction limit; this is achieved by using an apertured fibre optic scanning tip. The SNOM technique requires relatively high photon intensities such as those provided by an IR free electron laser (IR-FEL). The SNOM-IR-FEL enables the simultaneous collection of topography and optical features at scales not normally achieved with conventional IR techniques to produce high quality, chemically-rich images at designated wavelengths with a spatial resolution of ≥0.2 μm7,8.
Increased synthesis of proteins, lipids and nucleic acids is a condition for the rapid proliferation of cancer cells9, and changes in the bioavailability of these biomarkers can reveal important patterns of intracellular change. The IR-FEL on the ALICE accelerator at Daresbury Laboratory (Warrington, UK) is tuneable over the range of 5.5 μm to 8.8 μm (~1818 cm -1 to ~1136 cm-1), which includes a number of biologically important biomarkers10 at designated wavenumbers or wavelengths (Table 1). These biomarkers have previously been used to separate normal, low- and high-grade dyskaryosis and cancer cells from each other.
SNOM has been used to investigate the localisation of molecules within cell membranes of prostate cancer cells11. Further research demonstrated SNOM can accurately define both the cell surface and internal structures in both healthy and anomalous sperm, including the acrosome, nucleus and the organisation of mitochondria12, and has demonstrated potential for single molecule imaging13. The application of SNOM to oesophageal cancer tissue studies provided evidence of its potentiality for cancer diagnosis8. The increased spatial resolution of SNOM has the potential to reveal and quantify highly localised cancer-related changes in cervical cells at the sub-cellular level (1-0.1 μm), and more accurately and precisely than conventional IR techniques. The above-described IR-SNOM studies were all carried out in reflection mode. To our knowledge, this is the first publication reporting data obtained using IR-SNOM in transmission mode, and using the technique to image whole cervical cells.
The aim of this study was to assess the potential of SNOM in combination with an IR-FEL in the detection of the biophysical properties of cervical cell abnormalities. Spectra were also collected using traditional ATR-FTIR biospectroscopy to investigate the differences between techniques.
Results
We recruited 5 patients into this pilot study; the youngest aged 25 years (squamous & glandular pre-invasive lesions [CIN2, HGCGIN]) and the oldest 42 years (squamous lesion; low-grade dyskaryosis). Table 2 shows the characteristics for each patient; limited demography was available for the patient diagnosed with high-grade dyskaryosis.
Two out of the 5 patients were current smokers (high-grade dyskaryosis and CIN2 HGCGIN), and 1 patient was taking antibiotics (CIN2, HGCGIN). Four patients tested positive for HPV; none for HPV 18 and 2 for HPV 16. Both normal and high-grade dyskaryosis tested positive for HPV ‘other’ type (i.e., not high-risk HPV types 16 or 18); only normal had an abnormal high vaginal swab. All patients were tested for bacterial vaginosis and were normal.
A total of 34 cells were included in the SNOM images. The number of cells was evenly distributed for low-grade dyskaryosis, CIN2, HGCGIN and adenocarcinoma stage 1B1 (6, 5, and 5 cells each, respectively). Sixteen cells were imaged for normal and 2 for high-grade dyskaryosis, which was limited by the number of acceptable cells on the slide. Each SNOM scan comprised topographic, raw transmission (SNOM light) and IR (light) intensity reference images all collected simultaneously at a fixed wavelength. Example SNOM-IR-FEL topography and associated transmission images for the pre-invasive lesion (CIN2, HGCGIN) are presented in Fig. 1 (see Methods: Computational analysis). The topography and associated transmission images for the other 4 cells types is presented in Electronic Supplementary Information (ESI) Figures S1-S4.
Full Article
http://www.nature.com/articles/srep29494
Ultimi Articoli
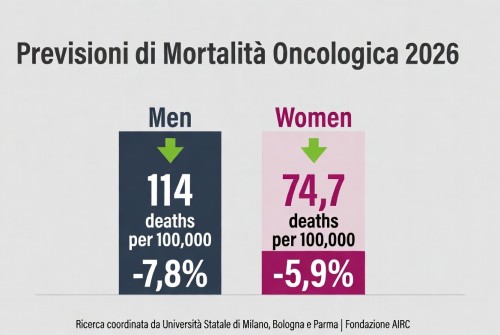
La mortalità per cancro cala in Europa – tassi in diminuzione nel 2026, ma persistono disparità

Carofiglio porta — Elogio dell'ignoranza e dell'errore — al Teatro Manzoni

Teatro per tutta la famiglia: “Inside and Out of Me 2” tra ironia e interazione

Dogliani celebra quindici anni di Festival della TV con “Dialoghi Coraggiosi”

Sesto San Giovanni — 180 milioni dalla Regione per l’ospedale che rafforza la Città della Salute

Triennale Milano — Una settimana di libri, musica, danza e arti sonore dal 20 al 25 gennaio

A febbraio la corsa alle iscrizioni nidi – Milano apre il portale per 2026/2027

Hackathon 2025 — a Palazzo Lombardia gli studenti sfidano il cyberbullismo

Firmato il nuovo Protocollo per il Punto Unico di Accesso tra Municipio Roma III e ASL Roma 1



