A new electrode material for rechargeable lithium batteries could mean smaller, lighter, longer-lasting laptops and cell phones.
Yet-Ming Chiang and colleagues at the Massachusetts Institute of Technology in Cambridge, Massachusetts, have made a substance called lithium phospho-olivine conduct electricity much better than the materials currently used as terminals in commercial batteries1.
"This may allow the development of lithium batteries with the highest power density yet," they say.
Until now sending current through lithium phospho-olivine has been like trying to send it through a lump of rock. "One of the main drawbacks with using these materials," said battery researchers Jean-Marie Tarascon and Michel Armand at the end of last year, "is their poor electrical conductivity.
Adding just one or two percent magnesium, aluminium, titanium or tungsten to lithium iron phosphate makes its conductivity rocket by about a million-fold, Chiang's team now reports.
So far, preliminary tests on batteries with electrodes made from this lithium phospho-olivine suggest that their storage capacity, and the amount of power that they can deliver, should be 10-20 percent greater per gram, than current lithium batteries.
In such a competitive industry, even a small improvement can make a big difference. Chiang and colleagues point out that such margins also improve the prospects of using lithium batteries in electric vehicles, where power and storage limitations are currently obstacles.
Philip Ball
Ultimi Articoli

Neve in pianura tra venerdì 23 e domenica 25 gennaio — cosa è realmente atteso al Nord Italia

Se ne va Valentino, l'ultimo imperatore della moda mondiale
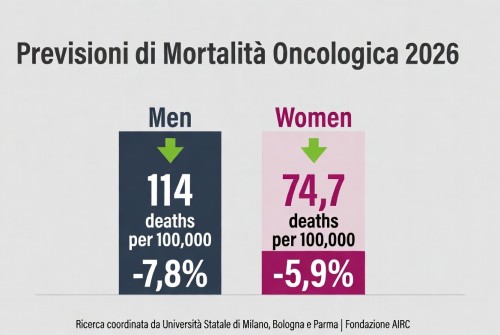
La mortalità per cancro cala in Europa – tassi in diminuzione nel 2026, ma persistono disparità

Carofiglio porta — Elogio dell'ignoranza e dell'errore — al Teatro Manzoni

Teatro per tutta la famiglia: “Inside and Out of Me 2” tra ironia e interazione

Dogliani celebra quindici anni di Festival della TV con “Dialoghi Coraggiosi”

Sesto San Giovanni — 180 milioni dalla Regione per l’ospedale che rafforza la Città della Salute

Triennale Milano — Una settimana di libri, musica, danza e arti sonore dal 20 al 25 gennaio

A febbraio la corsa alle iscrizioni nidi – Milano apre il portale per 2026/2027